A. Genetics - Tissue Culture - Cultivation
Our expertise in genetics positions Mandara Pharma for dramatic growth and builds our value
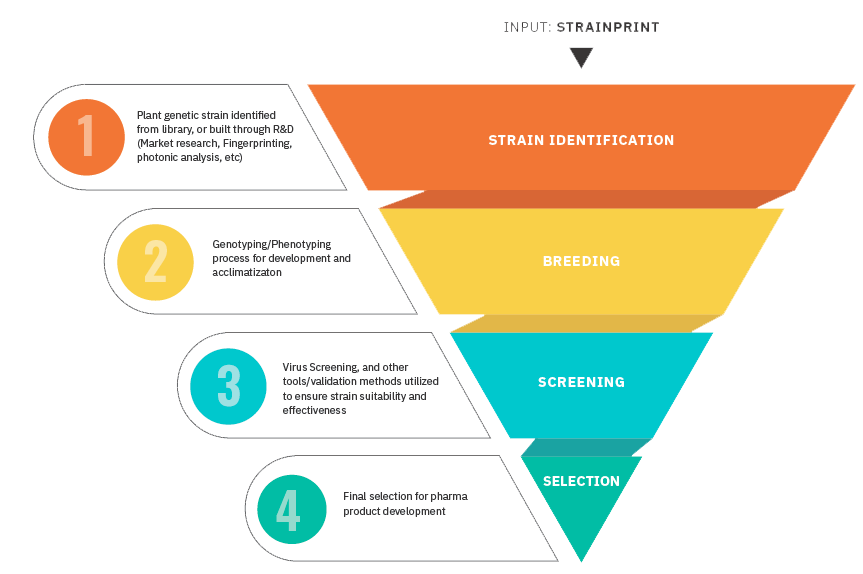
Our proprietary strains have advantages over those of the competition:
- Strong and disease-resistant
- Good match for drug development vs. specific therapeutic indications
- Significantly high CBD content compared to average market strains leading to a) 50% reduction in biomass requirements to produce the same quantity of full spectrum oil. b) 43% reduction in COGS, thereby significantly increasing the profit margin across a diverse product portfolio – and c) oncreased extraction and processing efficiencies and equipment availability.
Part of our quality assurance is the use of tissue culture plants in our cultivation program and produced in a laboratory environment as a sterilized plant which is then acclimatized prior to us growing the plant out in either a greenhouse or outdoor cultivation setting.
Our cannabis cultivation is strictly controlled by highly experienced growers who are trained in standard operating procedures (SOPs) as mandated by Health Canada’s Good Agricultural Practices (GAP).